As SARS-COV-2 Continues to Evolve, We've Got Your Back
SARS-CoV-2 is a rapidly evolving pathogen, and your team needs up-to-date data about whether your therapeutic provides protection against current variants. Reporter Virus Particles (RVPs) are a versatile and indispensable resource that can help you measure protection from neutralizing antibodies over time and across viral variants.SARS-CoV-2 RVPs are an off-the-shelf alternative to live virus, ideal for neutralization assays. Use RVPs to efficiently test your therapeutics and vaccines against all significant SARS-CoV-2 variants past and present. Best of all, as pseudoviruses, SARS-CoV-2 RVPs are safe for your BSL-2 lab.
Advantages over live virus
✔Safe in BSL-2
✔Ready to use
✔Convenient reporter, compatible with standard equipment
✔Quality controlled production for use as a critical reagent
Applications
✔Antibody neutralization
✔Serum screening
✔High-throughput assays
Product Offerings
Our RVP product catalog includes dozens of SARS-CoV-2 variant pseudoviruses ready to ship. Additional variants and custom products are available upon request for your COVID-19 therapeutic or vaccine development project.We are continually updating our catalog with the latest vaccine recommendations and SARS-CoV-2 variants of concern.
“SARS-CoV-2 RVPs really helped us a lot since we did not have much previous expertise in SARS. The user meetings were especially helpful.”
-YUFEI XIANG, UNIVERSITY OF PITTSBURGH SCHOOL OF MEDICINE
Reproducible, Specific SARS-CoV-2 Neutralization Data
Reporter Virus Particles (RVPs) provide highly reproducible and specific neutralization data to support your research.
Our ISO 9001-certified quality management system means you can count on reliable reagents that perform as expected in your lab. As the neutralization curves show (below left), there is minimal variation in neutralization data across RVP production lots. These six assays were run by different operators on different lots of RVPs over two years, resulting in a very consistent NT50 for the same antibody over time. Our careful attention to RVP production and critical quality attributes means you can focus on your research.
With SARS-CoV-2 RVPs, you can verify the specificity of your antibody and assess its ability to block infection. Below left, results from a neutralization assay show that RVPs faithfully present spike protein. Here, benchmark SARS-CoV-2 antibody VHH-72 specifically blocks infection by the expected SARS-CoV-2 RVPs, and not a mismatched control RVP. Moreover, as the concentration of the neutralizing antibody, in orange, increases along the x-axis, you see a neutralization curve from which it is possible to determine the neutralizing titer, or NT50.

(Left) Neutralization assay across 6 production lots of SARS-CoV-2 (D614G) RVPs show high lot-to-lot consistency in neutralization. (Right) SARS-CoV-2 RVPs are specifically neutralized by SARS-CoV-2–directed antibody VHH-72.
For more about neutralization assays, including tips
for developing your own assay, visit Pseudovirus
Neutralization Assays: A how-to guide for getting started.
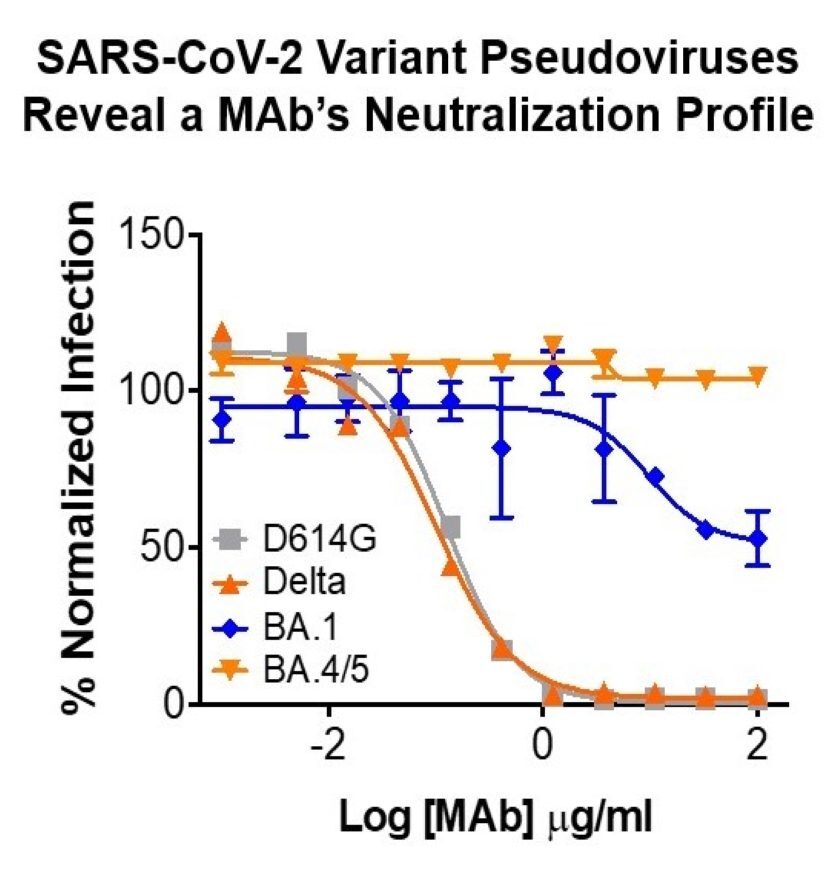
Integral Molecular’s broad catalog of SARS-CoV-2 variant pseudoviruses makes it easy to evaluate your antibody’s neutralization capability across ancestral and emerging variants.

SARS-CoV-2 will continue to persist as an endemic pathogen. The FDA has continued including pseudoviruses as important tools to measure virus neutralization in developing modified COVID-19 vaccines as well as to evaluate immune responses in vitro. Our development of SARS-CoV-2 RVPs in response to new variants gives researchers on-demand access to new strains as they emerge.
To learn more, visit How Pseudoviruses Meet FDA Guidances.
Featured Case Study
Learn how Immunome used SARS-CoV-2 variant pseudovirus to test their antibody cocktail
Monoclonal antibodies can provide efficacious therapy for COVID-19 treatment, but rapid mutation often leads to viral escape. Immunome developed a SARS-CoV-2 three-antibody cocktail with broad neutralization potency. They needed to test their antibody cocktail against a large panel of viral variants of concern.
Contact Us
Frequently Asked Questions
How do SARS-CoV-2 variant pseudoviruses replace live virus in an experiment?
Because it’s presented on the surface of an RVP, the SARS-CoV-2 spike protein maintains the same conformation it has on live virus. There is strong evidence to support that the native presentation of the spike results in highly comparable results between live virus and SARS-CoV-2 RVPs. See the following results published in peer-reviewed articles showing the close correlation between live virus and SARS-CoV-2 RVPs:
- Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2 Xiang et al. 2020 Science 370, 1479-1484
- A stapled lipopeptide platform for preventing and treating highly pathogenic viruses of pandemic potential Bird et al. 2024 Nature Communications 15, 274
- IMM-BCP-01, a patient-derived anti-SARS-CoV-2 antibody cocktail, is active across variants of concern including Omicron BA.1 and BA.2 Nikitin et al. 2022 Science Immunology 7, eabl9943
- A Phase I/II clinical trial of intradermal, controllable self-replicating ribonucleic acid vaccine EXG-5003 against SARS-CoV-2 Koseki et al. 2023 Vaccines 11, 1767
I’m ready to order. Which reporter gene should I choose?
How much SARS-CoV-2 pseudovirus should I order?
If you plan to run a large number of plates, we can produce large RVP lots, which can help avoid the need for bridging studies.
What cell line should I infect with RVPs?
What controls do I need for a neutralization assay with SARS-CoV-2 variant pseudoviruses?
Another control is one that tests the specificity of your antibody for SARS-CoV-2. We offer reporter pseudovirus expressing a VSV protein that will not be neutralized by antibodies that have high specificity for SARS-CoV-2.
These reagents make it easy to include reliable controls in your experiments. Our support team is also available to help you decide what control conditions you need for your assay.
For more information about establishing a neutralization assay, visit Pseudovirus Neutralization Assays: a how-to guide for getting started.

