Recapitulating biological processes | Time-saving & ready-to-use | Establishing an input volume | Designing your assay
| Safe, efficient & trusted | Contact us
Establishing your pseudovirus neutralization assay
Pseudoviruses can be used in place of live viruses for a variety of applications, including in viral entry assays, virus neutralization (VNT) assays, and microneutralization (MNT) assays. You’ve probably heard about pseudoviruses’ superb safety features and their convenient optical reporter. But starting a neutralization assay project can be daunting, and you may not know where to begin.
The information here will help you get started with an assay for moving your therapeutic antibodies or vaccine candidates forward—and help ensure your success.
While this page describes important considerations for neutralization assays using our Reporter Virus Particles (RVPs), the concepts are generally applicable to all pseudoviruses and virus-like particles.
If you need virus neutralization data for your antibodies or small molecules, consider using our Neutralization Assay Services. We have the facilities, the reagents, and the know-how to deliver quality data quickly and cost-effectively.
Pseudoviruses recapitulate the biological entry mechanisms of live viruses—safely
Integral Molecular’s RVPs have the antigenically correct viral entry and fusion proteins on their surface. That means you can use them in generating the important virus neutralization data required for advancing therapeutic antibody and vaccine candidates.
Instead of a viral genome, RVPs contain an optical reporter. Reporter gene expression offers a convenient readout for whether the particle has entered and fused with the host cell.
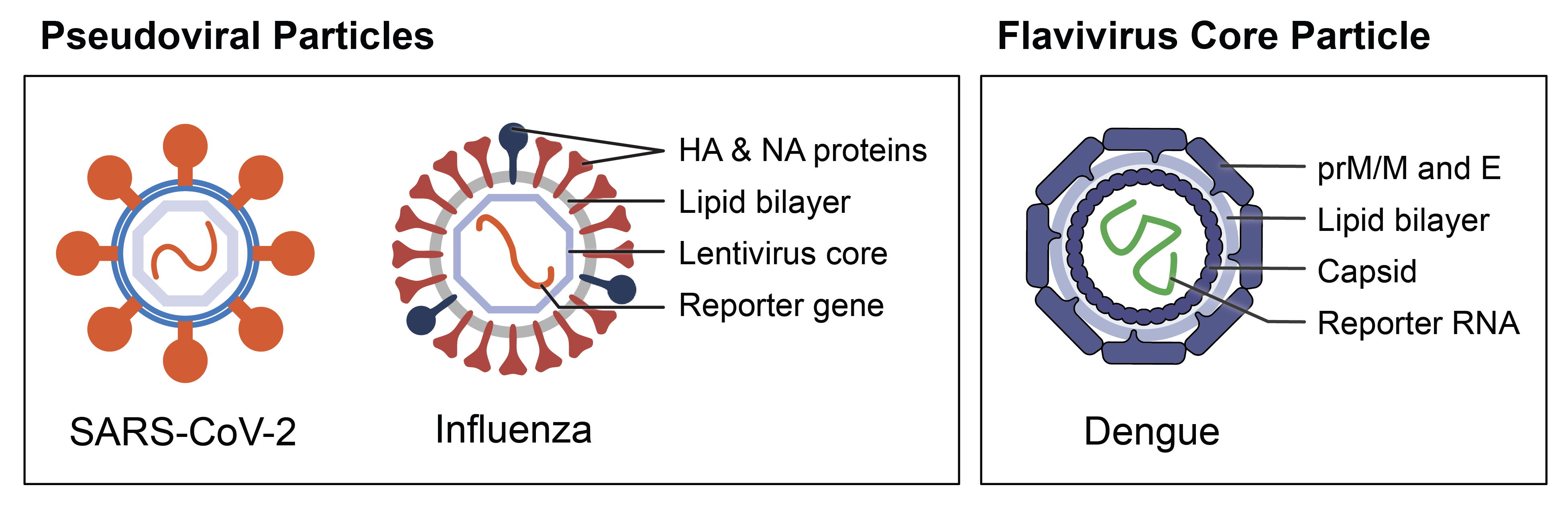
Reporter Virus Particles (RVPs) from Integral Molecular’s product offerings. Pseudovirus RVPs express viral surface proteins in a membrane surrounding a heterologous lentivirus core. They can express one surface protein (e.g., SARS-CoV-2 RVP) or multiple (e.g., Influenza RVP). Lentivirus core particles (e.g., Dengue RVP) have surface and structural proteins from a virus of interest, but they lack genes essential for replication. All RVPs contain a GFP or luciferase reporter.
Reporter Virus Particles are time-saving, ready-to-use products
- Protocols for the best possible neutralization assay results
- Recommended cell types, cell culture media, cell seeding densities, and washes or media changes. With many of our pseudovirus kits, you receive the cells and control antibodies you need.
- Known infectivity (GFP and luciferase) and titer (GFP) from our in-house quality control assay. Conducted on susceptible host cells, this infectivity assay can help you establish an input volume for your neutralization assay.
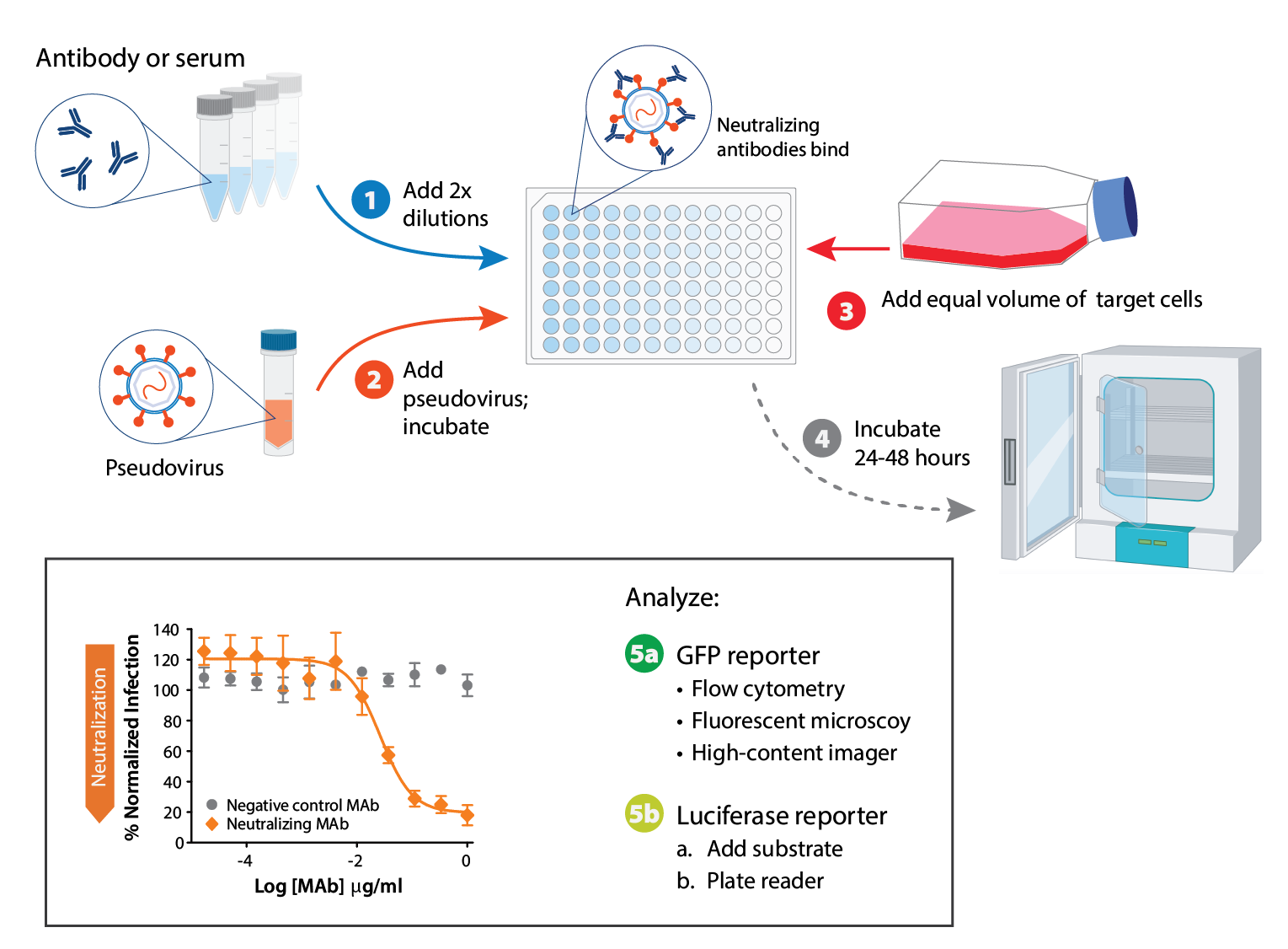
Pseudovirus neutralization assay at a glance. RVPs are available with a GFP or luciferase reporter. Reporter expression indicates when cells have been infected successfully. GFP read-out can be assessed by microscopy or flow cytometry, and luciferase readout can be assessed by plate reader.
Establish a pseudovirus input volume for your assay
You’ll want to choose an input volume for your pseudovirus neutralization assay that will give sufficient signal without using unnecessary amounts of product.
If you’re using RVPs, you may use the recommended input volume based on our QC assay, or you may choose to repeat the pseudovirus entry assays to account for any differences in practice between your lab and ours.
Typical inputs range from 2–10 microliters per well in a 96-well plate for RVPs, but the optimal volume can vary based on the virus and laboratory practices.
Choose an assay plate layout
An efficient layout will make your assay easier to set up.
96-well plate formats are popular for microneutralization assays, and they allow for a single 11-point dilution series in a horizontal layout. 384-well plate formats can allow three to four samples to be run in quadruplicate on a single plate. If you plan to automate your assays, make sure your plate layout will translate well to a robotic system such that tips for fluid handling do not have to be changed between samples.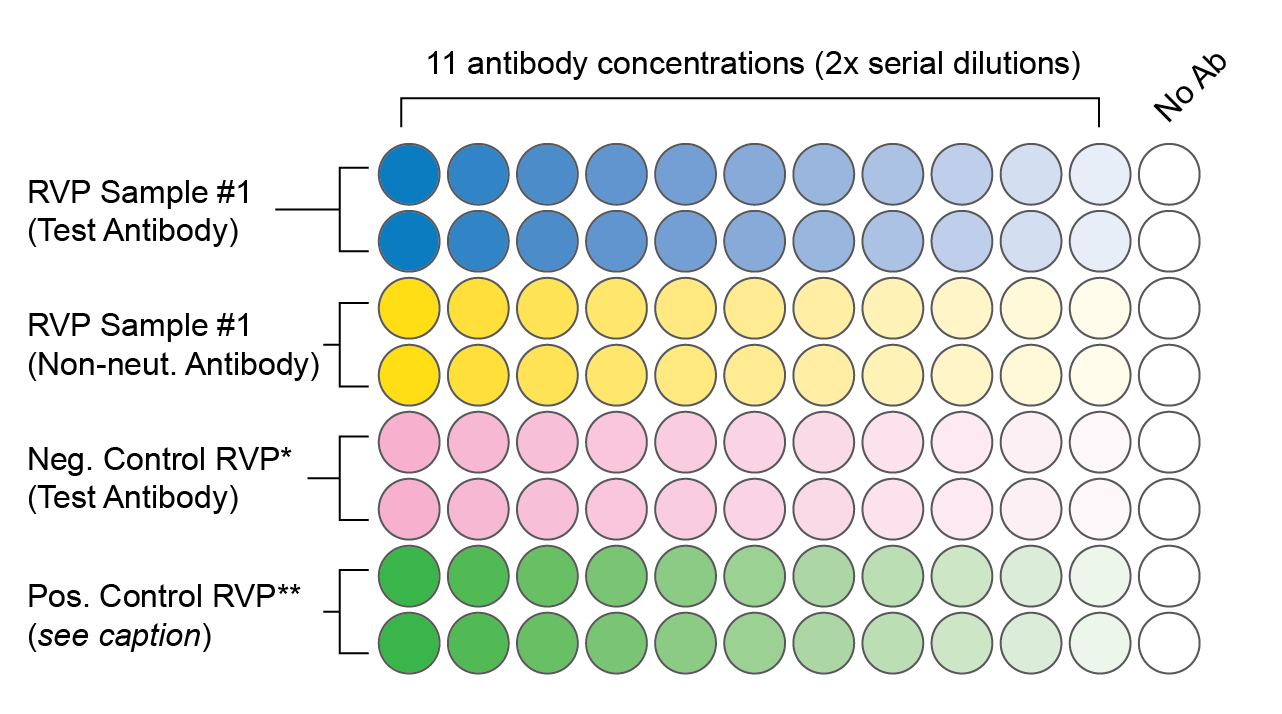
A sample 96-well plate layout that includes appropriate controls. Each condition is tested in duplicate at 11 antibody concentrations. The final volume in each well is 200 ul (50 ul serially diluted antibody or serum, 50 ul diluted RVPs, and 100 ul cells).
* An RVP that is known to evade neutralization by the test antibody. This controls for potential false positive results, e.g. due to cell toxicity.
** If available, an RVP that is known to be neutralized by either the test antibody or the non-neutralizing antibody. In the event of a negative result, this ensures that antibody was present and that there were no technical errors.
Design your neutralization assay
Neutralization assays for sera samples
Ensure that you have access to the rigorous positive controls required to validate your assay results.
World Health Organization (WHO) reference samples are available from NIBSC. These samples are intended primarily as a reference standard for serum neutralization assay harmonization and comparison between labs (Mattiuzzo et al., 2019).
Because WHO reference standards are very valuable and intended for use by labs worldwide, they are not intended for single-plate controls. Instead, as a positive control for neutralization, you can spike a negative serum sample with a known positive serum sample. By calibrating your known serum sample to a shared reference standard, you can ensure that all your assay plates are appropriately harmonized and your data can be compared across days and with other labs referencing the same standard (e.g., Bonhomme et al., 2022; Benkeser et al., 2023).
Establish a dilution series for your samples.
Serum samples contain a mixture of antibodies, including neutralizing and non-neutralizing antibodies. It’s important to determine an appropriate dilution factor for the serum to ensure that the neutralizing antibodies are present at a concentration that allows their activity to be accurately measured.
To empirically determine the amount of serum that should be used, start with a wide dilution range over a sub-set of samples, then narrow the dilution range if needed.
Account for non-specific binding and interference.
Serum samples contain components aside from IgG antibodies that can potentially interfere with the assay or cause non-specific binding to the pseudovirus or assay components. This non-specific binding may lead to false-positive results for neutralization.
Appropriate controls, such as serum from uninfected individuals and negative controls without serum, should be included in every assay to account for background interference.
Data generated from a well-controlled pseudovirus neutralization assay can be used to qualify your assay. Measurements such as robustness, limits of quantitation, dilutional linearity, precision, and accuracy should all be part of your assay qualification data.
Neutralization assays for your antibody therapeutic
Establish compatible quality specifications for antibody production.
For example:
- Avoid azides. Azides are sometimes used as a preservative in antibody preparation, but they are toxic to live cells. Thus, they may affect the results in functional assays—including neutralizing antibody tests (Weyermann et al., 2005).
- Ensure that your antibody is sufficiently concentrated to accommodate the necessary range of concentrations in your neutralization assay. We recommend something in the range of 200 ug/mL. Ideally, antibodies used in neutralization studies should be affinity purified.
Determine your antibody’s affinity
- Use your antibody affinity data to help you determine a target concentration range for your pseudovirus neutralization assay. The affinity of the antibody for viral proteins may be the same order of magnitude as the IC50 (e.g., Pymm et al., 2021). If you do not have affinity data, screening a broad range of concentrations using 3-fold dilutions from concentrated antibody can help you determine a range to use for additional neutralization assays.
Antibody concentration is a critical factor in setting up neutralization assays for antibody therapeutics. Establishing an optimal range will save time and reagents.
Through our antibody Neutralization Assay Services, we can determine all necessary starting parameters for you. You provide antibodies and any known affinity data for your antibody with a known viral receptor, and we provide reliable, rapid data. Depending on the nature of the project—including the virus of interest and number of antibodies—the turnaround time ranges from 4-12 weeks. To learn more, use the contact us form, below.
Reporter Virus Particles are safe, efficient, and trusted
Virology researchers who study dangerous pathogens not only invest their time, they also take on personal risks in their quest to make the world a safer place. We strive to provide efficient, effective, up-to-date tools for advancing research in the safest way possible.
Importantly, our products can save your team’s valuable time. Growing live virus can mean pulling the best members of your team to grow, purify, and aliquot live virus. With ready-to-use pseudoviruses, your team can instead focus on key analytical tasks.
Our pseudoviruses for influenza, SARS-CoV-2, SARS-CoV-1, filoviruses, and Nipah virus are trusted throughout the industry. For links to publications and more, visit the Resources page. Our flavivirus core particles for dengue and Zika viruses have been well-characterized in peer-reviewed publications and cited by major vaccine manufacturers. For an example, read this case study featuring work from Takeda.
View our selection of products and order today. Be sure to check out our growing selection of RVP Neutralization Kits, with everything you need to get started with your own pseudovirus neutralization assay. Beyond the items in our catalog, Custom Reporter Viruses are available to meet your specific need.
Case Studies
Contact Us


