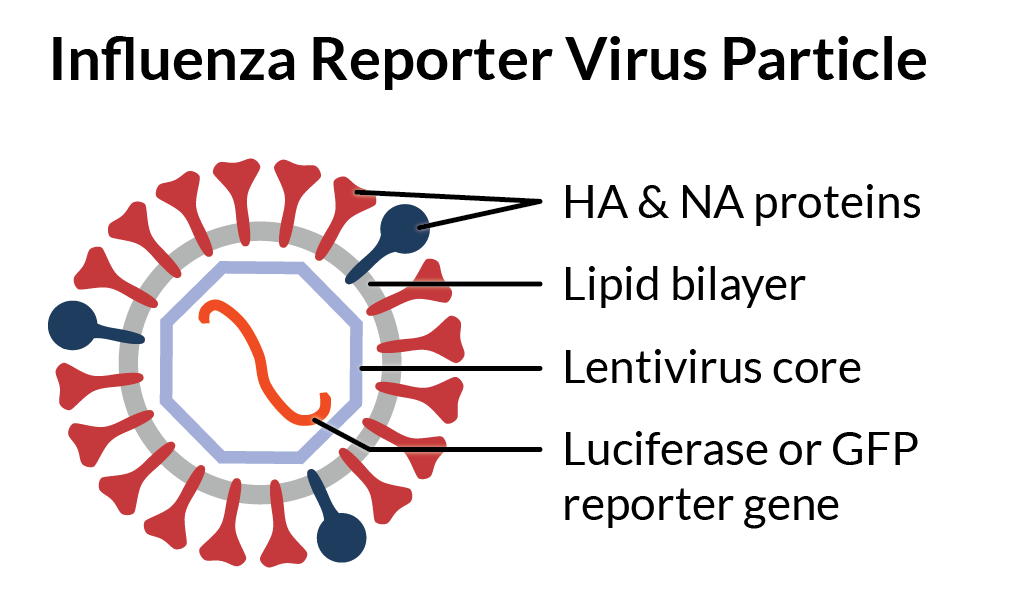
Influenza A & B Pseudotyped Viral Particles
RVPs display strain-specific hemagglutinin (HA) and neuraminidase (NA) proteins on a heterologous viral core. Their modified genome expresses a convenient GFP or luciferase reporter gene that produces a quantitative readout of infectivity that’s easy to detect on standard instruments.
Unlike live influenza virus, Influenza RVPs are produced by direct expression of viral proteins encoded by cDNA. This means the product is the same every time, and we can easily modify the HA sequence to suit your research needs.
With this reliable, off-the-shelf reagent, your lab team will spend less time sourcing up-to-date influenza stocks, propagating virus, and training personnel—leaving more time for generating valuable data in response to the latest influenza strains.
Common Applications
✔Antibody neutralization testing
✔Serum screening and potency assays
✔High-throughput screening campaigns
Advantages over live virus
✔All strains are BSL-2 safe
✔Quantitative readout without staining
✔Point mutations or novel strains in weeks
Need a custom strain or mutation? Through our custom pseudovirus service we can create influenza virus particles tailored to your specific needs.
Related product: Influenza TiterSafe™ Particles are a ready-to-use reagent that readily substitutes for live influenza virus in your existing hemagglutination inhibition (HAI) assay. It’s a time-saving, safer alternative for vaccine developers running traditonal potency assays.
Consistent & Easy Influenza Neutralization Assays
Our ISO 9001-certified quality management system means you can count on reliable reagents that perform as expected in your lab. As shown below (left panel), influenza RVP infectivity data is highly consistent across production lots. Our careful attention to critical quality attributes means you can focus on your research, not reagent troubleshooting.
With Influenza RVPs, you can verify your antibody’s specificity and assess its ability to block infection. The neutralization data below (right panel) shows that our influenza pseudoviruses faithfully present influenza surface proteins and generate clean dose-response curves for calculating NT50, IC50, or other metrics..
Ready to establish your own assay? Our guide walks you through the process: Pseudovirus Neutralization Assays: A how-to guide for getting started.
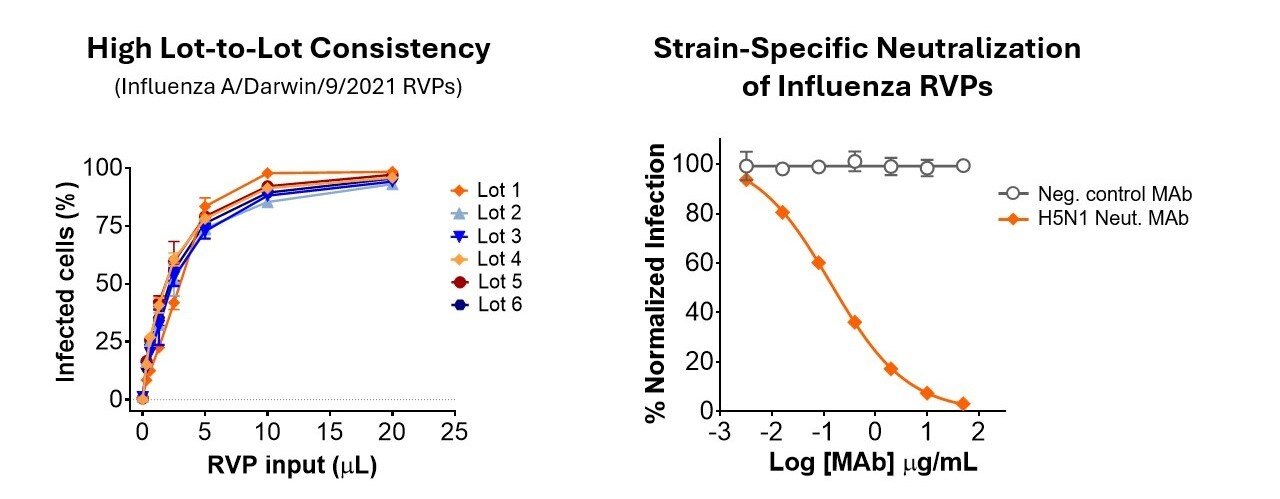
(Left) Infectivity assays comparing lots of Influenza A/Darwin/9/2021 RVPs were performed in 384-well plates. MDCK-SIAT1 cells were infected for 1 hour, then incubated for 72 hours prior to readout by GFP fluorescence. (Right) Infection of HEK-293T cells with Influenza A/Indonesia/5/2005 RVPs was inhibited by a neutralizing monoclonal antibody (Sino #68031-H011). A non-neutralizing control antibody did not inhibit infection.
Influenza Pseudovirus Neutralization Assay Workflow
Protocol overview
Assay formats we support
Do you have an alternative assay format or need a different readout? Please contact us for more information.
Featured Webinar: Innovative Research Tools to Combat Biological Threats
Contact Us