Get Ready for Easier Influenza Assays
With Influenza TiterSafeTM Particles, you can get straight to work testing influenza immune sera. The first product of its kind, Influenza TiterSafe is an off-the shelf reagent that can easily substitute for live virus in your existing agglutination assays.
Free from the chore of growing influenza virus stocks, your team will have more time to focus on important science. Plus, because Influenza TiterSafe Particles are non-replicative, your lab team will be safer.
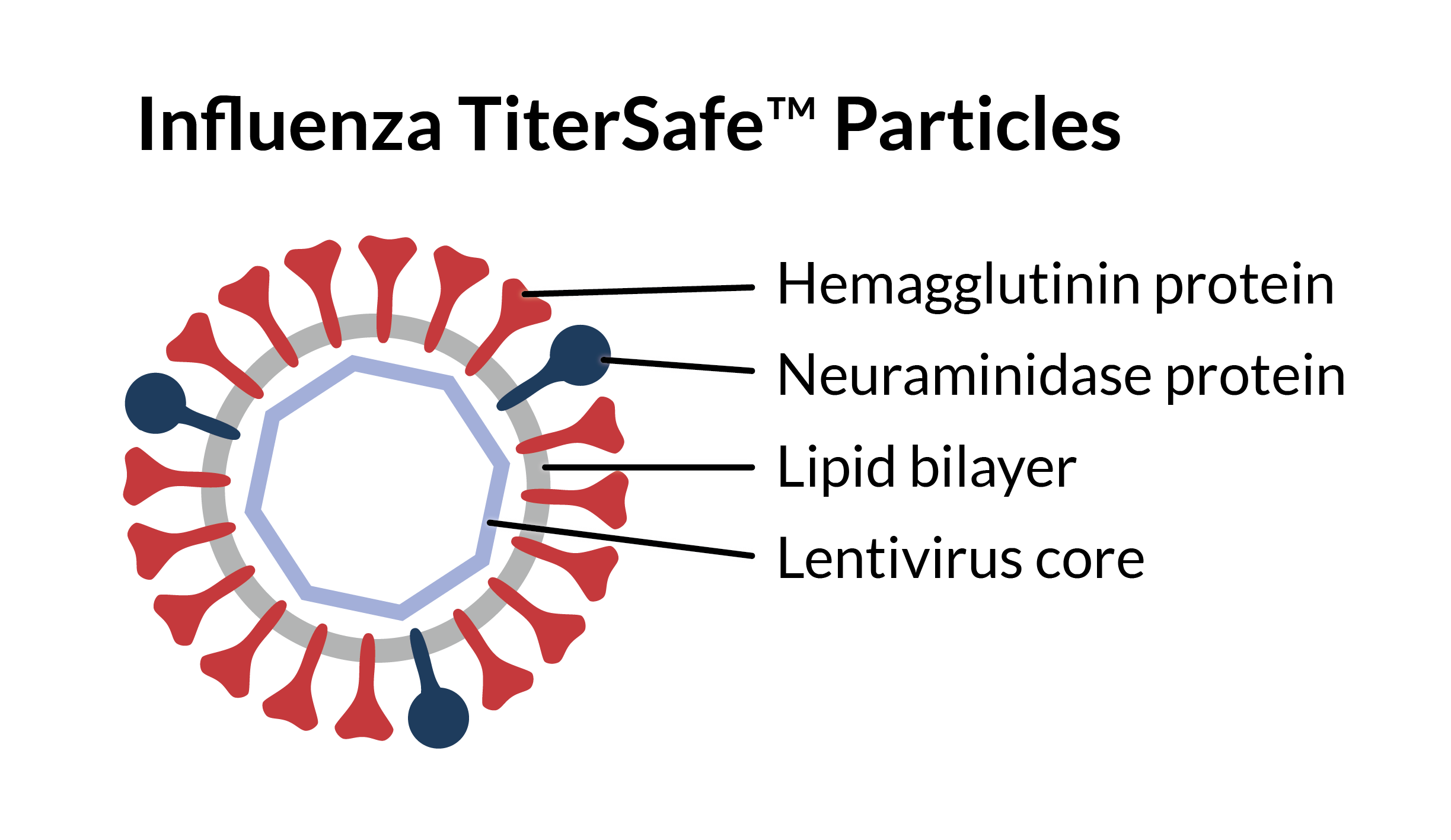
Influenza TiterSafe is a novel influenza virus-like particle designed for hemagglutination inhibition assays. It consists of membrane-wrapped particles with a lentivirus core. Its hemagglutinin (HA) and neuraminidase (NA) surface proteins have amino acid sequences identical to those from specific strains of influenza A or influenza B virus. Influenza TiterSafe Particles have many advantages over live influenza virus in HAI assays.
TiterSafe is a trademark of Integral Molecular.
Advantages
✔Ready-to-use, quality controlled agglutination reagent
✔Non-replicative & safe for BSL-2
✔Easy to substitute in your existing HAI assays
✔Available for seasonal & custom influenza strains
Influenza TiterSafe Product Offerings — Buy HAI Assay Reagents
* WHO-recommended strains for cell-based/recombinant vaccines
| Catalog No. | Virus (Subtype) | Strain Information |
|---|---|---|
| HAP-1302 | Influenza B (Victoria) | Austria/1359417/2021 |
| HAP-1303 | Influenza B (Yamagata) | Phuket/3073/2013 |
| HAP-1205 | Influenza A (H5N1) | Vietnam/1203/2004 |
| HAP-1212 | Influenza A (H3N2) | Darwin/6/2021 |
| HAP-1213 | Influenza A (H1N1) | Wisconsin/67/2022 |
| HAP-1214 | Influenza A (H5N1) | American Wigeon/SC/22-000345-001/2021 |
| HAP-1215 | Influenza A (H3N2) | Massachusetts/18/2022 |
| HAP-1216 | Influenza A (H3N2) | Thailand/8/2022 |
| HAP-1217 | Influenza A (H5N1) | Dairy_cattle/Texas/24-008749-003-original/2024 |
| HAP-1218 | Influenza A (H5N1) | Texas/37/2024 |
Related product: Influenza Reporter Virus Particles (RVPs) for safe, easy neutralization assays are available with a GFP or luciferase reporter gene. RVPs are available in seasonal and pandemic influenza strains; see Influenza RVP Products for details and to order.
Contact us today for a quote or a sample. Join our mailing list to receive new product announcements and more.
What Are Influenza TiterSafe Particles?
Influenza TiterSafe is a flexible influenza assay product designed for hemagglutination inhibition assays, also known as HAI assays, HIA assays, or HI assays. These influenza virus-like particles can also be used in other influenza assays, including hemagglutination (HA) assays, and neuraminidase enzyme-linked lectin assays (NA ELLA).
Influenza TiterSafe is based on our highly validated platform for building pseudotyped Reporter Virus Particles. TiterSafe is produced in human cells using transfected DNA encoding the influenza HA and NA proteins. Its HA and NA surface proteins have amino acid sequences identical to those in live virus.
Influenza TiterSafe Particles’ HA and NA surface proteins show the expected interactions and activities. In HAI assays with both sera and MAbs, TiterSafe shows the expected specificity for strain and lineage.
Because TiterSafe is not amplified by replication, the HA on the surface is the same on every particle and in every tube of reagent. Unlike with live virus, there is no sequence drift.
Due to its modular design, TiterSafe is flexible and highly customizable. By swapping out the DNA sequences, we can quickly and easily make TiterSafe product lines for new and custom influenza strains.
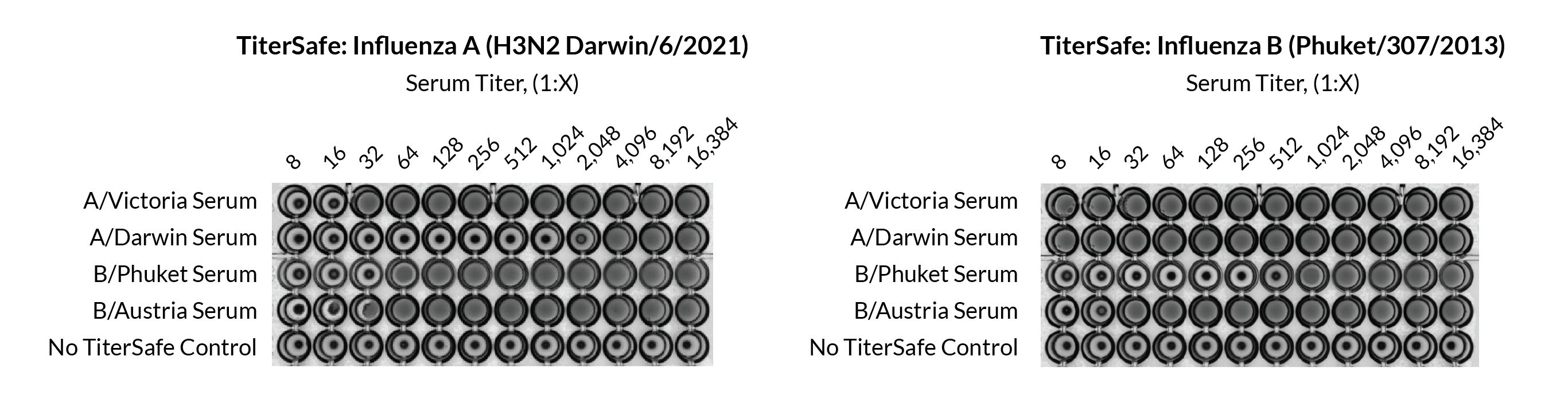
In sera and antibody screening, TiterSafe shows the expected specificity for strain and lineage.
How TiterSafe Replaces Live Influenza Virus
In hemagglutination inhibition (HAI) assays, Influenza TiterSafe Particles perform like live influenza virus by cross-linking red blood cells (RBCs). This cross-linked matrix is visible to the eye as a broad red area spread across the sample well. In the absence of cross-linking, or agglutination, the RBCs form a concentrated spot at the bottom of the well.
As with live virus, you can use Influenza TiterSafe to detect agglutination-inhibiting antibodies in vaccine sera, and thus estimate the level of protection against influenza virus. When specific antibodies bind to TiterSafe surface proteins, they block agglutination, and the RBCs form a concentrated spot.
Influenza TiterSafe is an easy and safe substitute. It fits into conventional HAI workflows as a straight replacement for live influenza virus. It provides accurate HAI assay results, ensuring that your research remains reliable and valid.
Because TiterSafe has a much better safety profile than live influenza virus, it can be easier to work with. It is non-replicative and safe in a BSL-2 environment.
Resources
To hear our customers describe how they have used Influenza TiterSafe Particles and Influenza Reporter Virus Particles to analyze the performance of therapeutic antibodies, view the webinar Innovative Research Tools to Combat Biological Threats
Visit the Resources page for more webinars, publications, and case studies featuring additional virology products and services.
Contact Us
Frequently Asked Questions
How do Influenza TiterSafe Particles save me money?
Does Influenza TiterSafe contain genetic material?
How do Influenza TiterSafe Particles compare to attenuated influenza strains?
How quickly will new Influenza TiterSafe strains be available after seasonal influenza strains are announced?
Because strains can behave differently in the lab, it’s difficult to put a timeline on the development of new products. However, we can produce TiterSafe Particles as fast as or faster than live virus. We aim to have new TiterSafe seasonal influenza strains available within 8-10 weeks of the seasonal strain sequence releases for the recommended Northern and Southern hemisphere strains, providing you with the HAI product you need for quickly testing influenza immune sera.
Keep in mind that Influenza TiterSafe Particles are concentrated and ready to use. That means that once TiterSafe arrives in your hands, you can start work in lab. There is no growing of banking of virus required.
How are Influenza TiterSafe Particles different from Reporter Virus Particles?
Influenza TiterSafe Particles are similar to Influenza Reporter Virus Particles (RVPs), but it lacks a reporter gene, and it is more concentrated.
Due to the similarities between the platforms we use to make the two products, we can provide matched reagents with the same surface proteins and overall structure: RVPs for neutralization assays, and TiterSafe for agglutination inhibition assays.
Can I order custom Influenza TiterSafe strains?
Do TiterSafe Particles have a standardized concentration?
- The dilution for 1 HA unit is at least 1:256.
- Each tube contains at least enough product for 1 HA plate and 3 HAI plates using our recommended protocol.
For example product sheets and protocols, please view the individual product page for the relevant strain or contact us.

