Your Fastest Microneutralization Assay
Ready Reporter Virus (RRV) kits offer a powerful blend of speed and biological relevance that’s unlike anything you’ve used before, all in a convenient thaw-and-use package. Results are ready in hours—10 times faster than traditional PRNT assays, which read out in days. Now you can generate neutralization data any day of the week.
Unlike binding assays or cell-free fusion assays, RRV kits use live cells and authentic viral antigen to generate functional data. The kits’ thaw-and-use target cells eliminate the need for cell culture and sterile technique, maximizing efficiency and eliminating the risks associated with passaging your own cells. The kits’ non-replicative viral particles quickly activate an optical reporter upon cell entry, eliminating the lengthy wait for gene expression.
RRV kits work seamlessly with standard equipment and automated systems, making them perfect for high-throughput environments. Because they require minimal equipment and training, RRV kits make efficient use of your team’s time.
Features
✔ Same-day results
✔ No tissue culture
✔ Real antigen & live cells
✔ Safe in BSL-2
✔ Easy to automate
Product Offerings — Buy Neutralization Assay Kits
Ready Reporter Virus (RRV) kits are available now for select SARS-CoV-2 variants and influenza strains. Stay tuned for new RRV kit releases and additional optical readout options. Contact us any time for more information or to join our mailing list.| Catalog No. | Virus (Subtype) | Variant or Strain | Target Cells |
|---|---|---|---|
| RRK-702 | SARS-CoV-2 | D614G | HEK-ACE2 |
| RRK-1701 | SARS-CoV-2 | JN.1 [16MPLF] | HEK-ACE2 |
| RRK-1207 | Influenza A (H5N1) | Vietnam/1194/2004 | HEK-293T |
| RRK-1215 | Influenza A (H3N2) | Massachusetts/18/2022 | MDCK-derived |
| RRK-1218 | Influenza A (H5N1) | Texas/37/2024 | HEK-293T |
| RRK-1001 | Vesicular Stomatitis Virus | Indiana | HEK-293T |
| RRK-1901 | Lassa | Josiah | HEK-293T |
Follow the link below to view individual product details and order an RRV Kit today. For a limited time, you can request a free sample.
How Ready Reporter Virus Kits Generate Biologically Relevant Results Safely
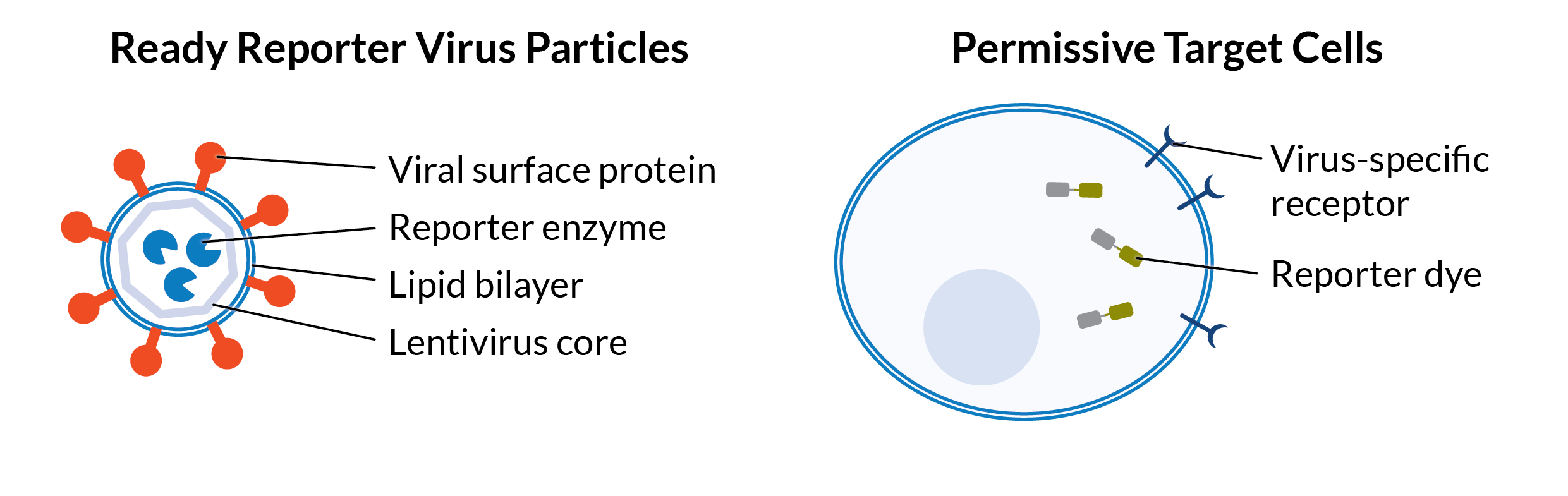
Ready Reporter Virus (RRV) kits use a pseudovirus system that models authentic viral entry in the absence of viral genetic material. Each kit includes non-replicative, pseudotyped reporter virus particles that display relevant viral envelope proteins, along with permissive target cells pre-loaded with reporter dye. When the viral particles fuse with the cell membrane, they deliver a reporter enzyme. The enzyme triggers a change in a fluorescent substrate inside the cell (luminescent substrates in development). This optical signal allows you to quantify neutralizing activity in test samples.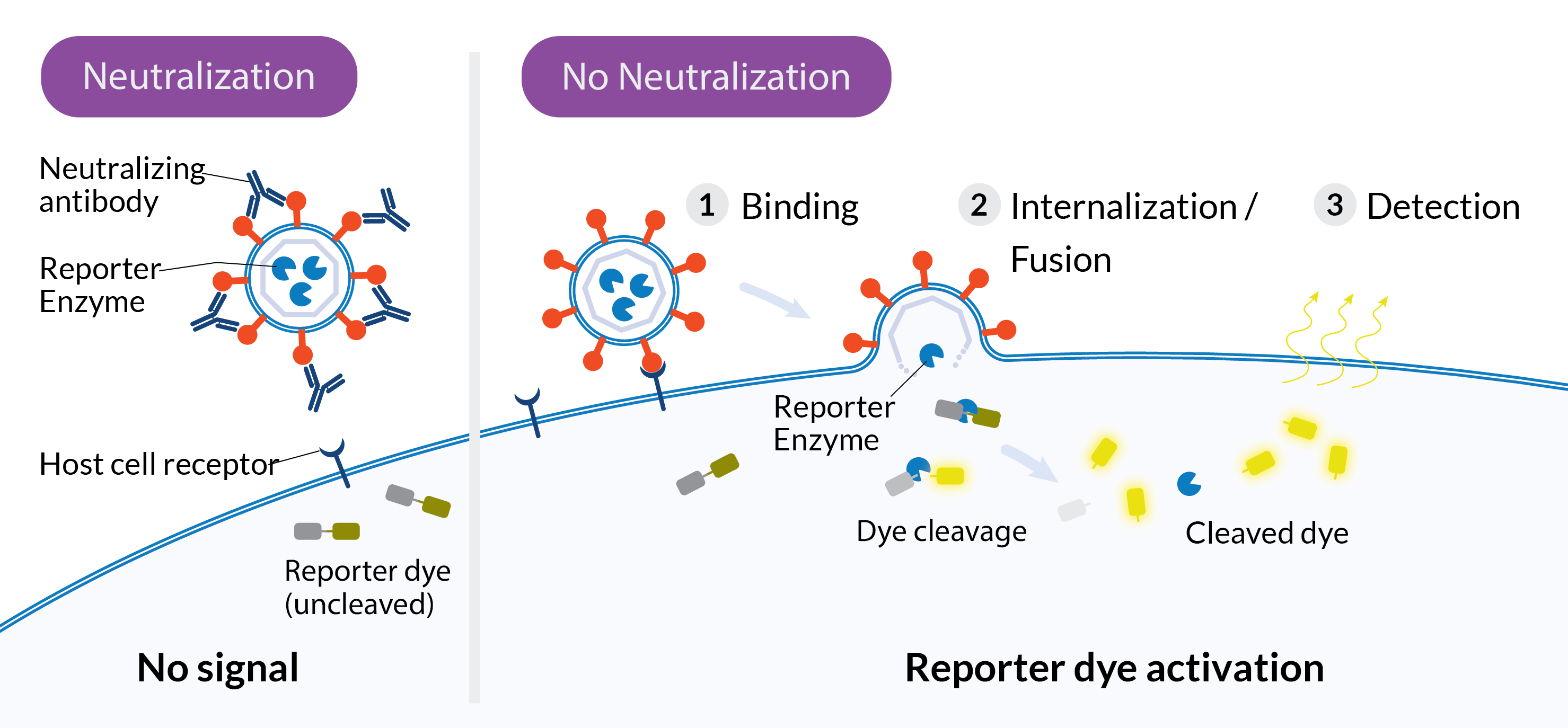
Reliable Results for Viral Entry Inhibition
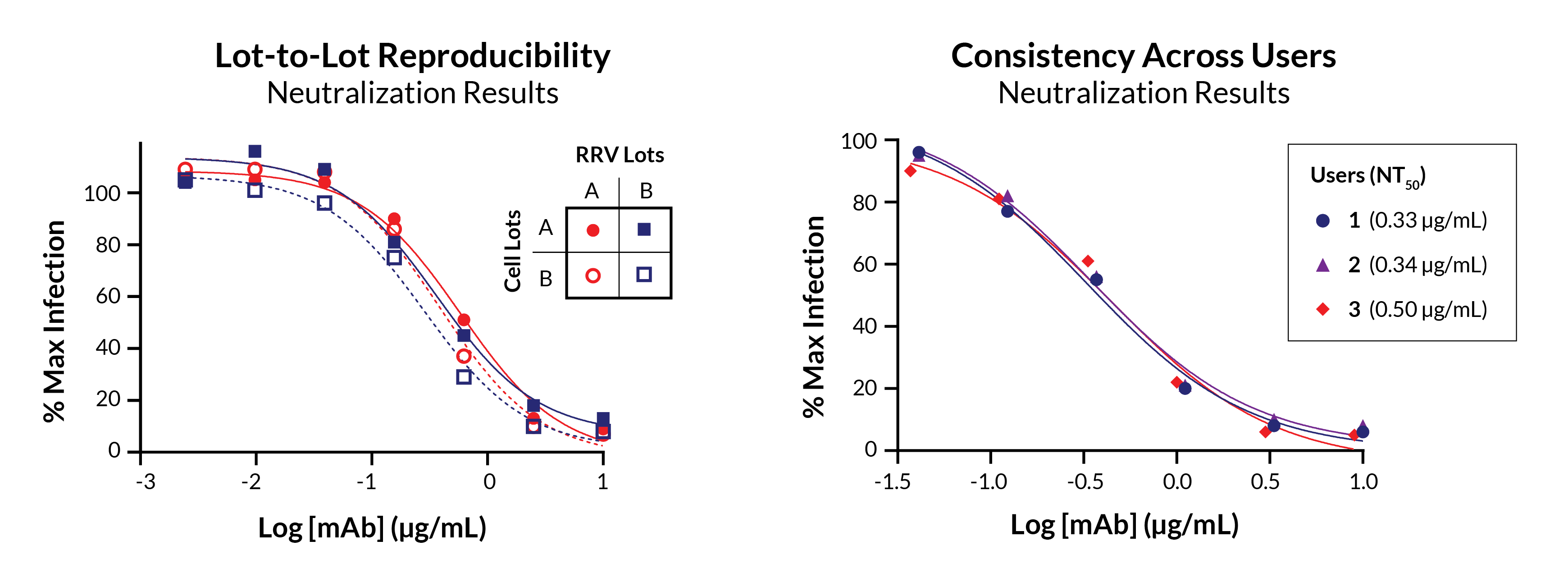
Beyond speed benefits, Ready Reporter Virus (RRV) kits deliver exceptional reproducibility. Demonstrated in the graphs below, RRV kits generate similar neutralization curves from lot to lot (left) and between users (right). We observe similar neutralization results from different users working on different days. Notably, these users differed in their experience levels, ranging from a PhD scientist to a junior research associate with minimal training.
Our ISO 9001 certified quality systems ensure the consistency and reliability you need to support vaccine development, antibody discovery and development, and basic research. RRV kits are built upon decades of expertise in virology research, product development, and customer service. Our pseudotyped Reporter Virus Particles have already been adopted by over 100 labs worldwide for research on diverse pathogens.
Example Applications Across Vaccine Development, Antibody Discovery, and Research
Vaccine Development
- Accelerate go/no-go decisions with fast neutralization readouts
- Reduce overhead by eliminating cell culture
and BSL-3 containment - Streamline workflows with automation
compatibility
Academic Research
- Maximize output with lean teams and minimal personnel
- Access expert support whether you’re new to virology or have advanced needs
- Focus on research, not assay optimization
Antibody Discovery
- Generate functional screening data for B-cell clones & antibody variants with minimal setup
- Support early-stage lead selection and potency ranking
- Use one assay from lead discovery through lot release testing
Application Highlight: Accelerating Antibody Discovery and Development
Ready Reporter Virus (RRV) kits streamline functional screening workflows for antibody discovery, enabling rapid, biologically relevant assessment of neutralizing activity. By eliminating cell passaging and reducing assay time to under a day, RRV kits enable function-forward screening for single clones. They allow researchers to test more candidates, iterate faster, and make lead decisions earlier in the development cycle.
Designed for standard lab equipment and automation platforms, RRV kits support high-throughput screening across multiple sample formats: B-cell supernatants, hybridoma libraries, engineered antibody variants, bacterial extracts, phage display, and more. The functional readouts provide critical data for lead selection, GMP potency assays, and regulatory submissions, making them an ideal tool for biotech teams focused on therapeutic innovation and speed-to-market.
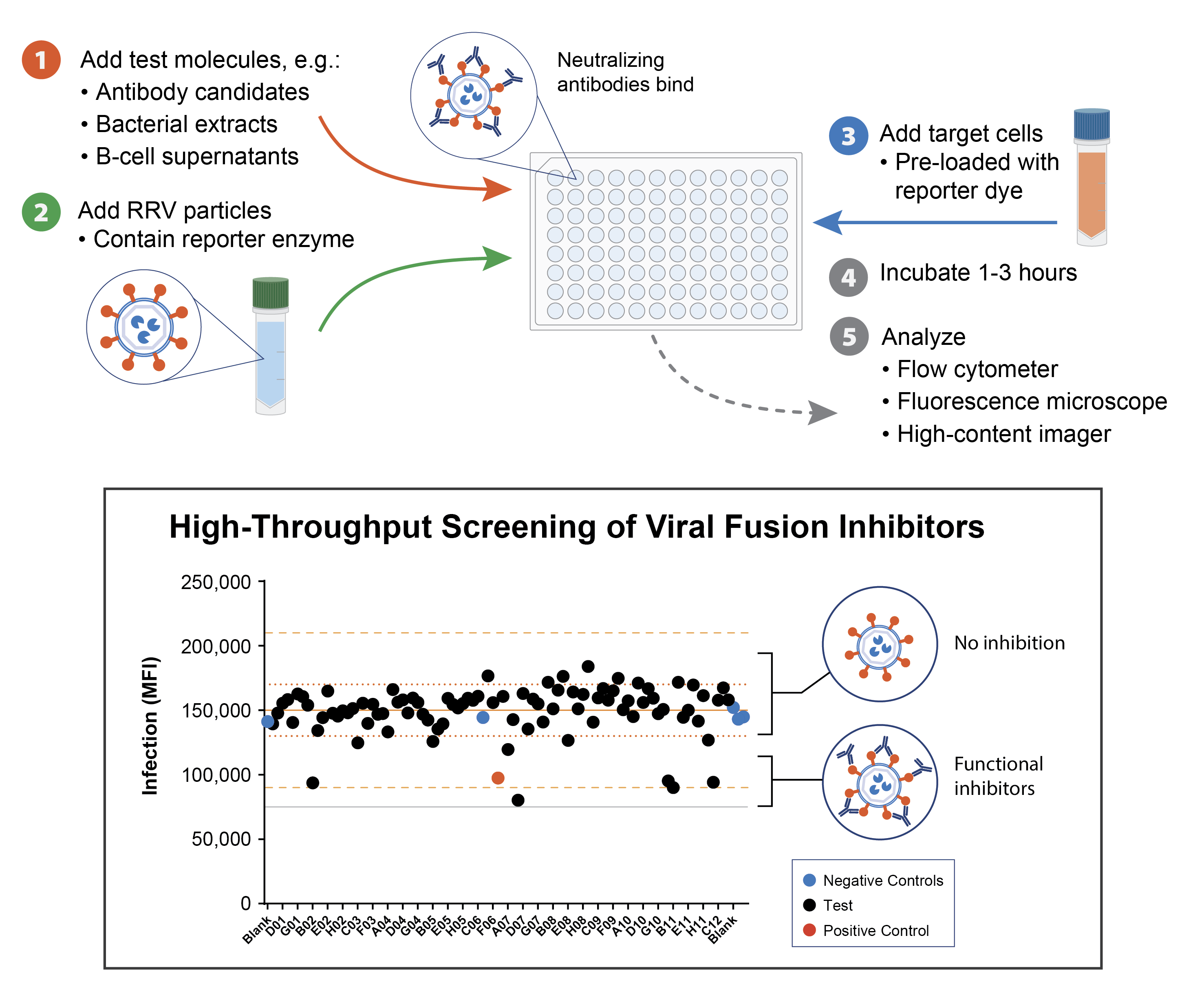
A SARS-CoV-2 RRV kit was used to screen numerous scFvs from E. coli for viral fusion inhibition. Gray line shows fluorescence background level. Solid orange line represents the mean, dotted lines show one standard deviation, and dashed lines show three standard deviations.
Frequently Asked Questions
How do I get a sample kit?
I don’t see what I need in your product catalog. Can you provide custom kits?
Do the Ready Reporter Virus particles contain a genome?
Can I use the Ready Reporter Virus kit to assess serum samples for neutralization?
Yes, the RRV kit was designed for testing human and animal serum samples. We recommend conducting any typical processing steps, such as heat inactivation, just as you would for your standard neutralization protocol.
The graph below shows sample data for neutralization of SARS-CoV-2 D614G RRV particles by human serum samples. Serum from vaccinated and naturally infected humans show expected neutralization profiles.
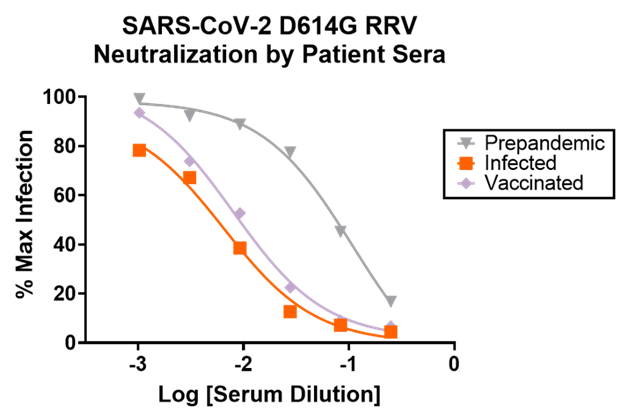
How does this assay fit into my current workflow and save me money?
Ready Reporter Virus (RRV) kits accelerate neutralization workflows by eliminating cell culture and reducing assay time to under 6 hours. Because the kits require no tissue culture, they minimize challenges from contamination, labor intensity, human error, resource management, and personnel training. Each kit uses pseudotyped, non-replicative viral particles that deliver a reporter enzyme upon membrane fusion with thaw-and-use target cells, enabling quantification of viral entry or viral fusion inhibition.
In a mathematical model of a 1,000-sample seroprevalence study, RRV kits were projected to reduce hands-on labor needed by 200 hours—a 50% reduction—compared to traditional pseudovirus assays. These efficiencies largely stem from the kit’s ready-to-use cells, which reduce total reagent costs, lower repeat rates due to tissue culture failures, and streamline workflows. Relative to PRNT assays, the kits have even greater savings: they generate results 10x faster, and they avoid labor costs for banking viral stocks, as well as risk of virus stock failure.
RRVs support reproducible NT50 determinations across users and lots, making them ideal for high-throughput screening of neutralizing antibodies and functional assays targeting viral entry and fusion. For labs transitioning from PRNT or RVP workflows, RRV kits offer a biologically relevant, cost-effective alternative without compromising data quality.
What laboratory supplies and equipment will I need to run the Ready Reporter Virus neutralization assay?
Protocols detailing required supplies and equipment are linked from individual product pages. The kits include Ready Reporter Virus particles, target cells, and signal enhancer. Each kit includes enough product for one 96-well plate, and it arrives frozen on dry ice.
In addition to test antibodies or serum samples, you will need to supply the following:
Supplies- 1.5 mL tubes
- 15 mL conical tubes
- Reagent reservoir
- 96-well U-bottom and V-bottom plates, plus lids or plate seal
- HBSS++ (HBSS + 10 mM HEPES + 0.5% BSA)
- Positive and negative control antibodies* or sera
* For some kits, our product catalog includes virus-neutralizing antibodies that can be purchased separately for use as positive and negative controls. Links to appropriate control antibodies, when available, are included on individual product pages.
Equipment- Freezer at -80°C (+/- 10°C) for storing kit before use
- Water bath at 37°C
- Incubator at 37°C
- Multi-channel and single-channel pipettors
- Plate shaker (optional)
- Flow cytometer (ex. 409nm; em. 447/520nm) for fully supported readout, or high content imager (ex. 409nm; em. 447/520nm; compatible but not yet validated)
Can I use a plate reader for this assay?
Can I use a high content imager for this assay?
Does the assay require wash steps?
How can I access technical support?
Contact Us
